Microscopic description:
On H&E sections, tumor cells show vascular channels lined with endothelial cells that show varying degrees of pleomorphism, mitotic activity and prominent nuclei.
On immunohistochemical studies, tumor cells show positive staining for CD31, ERG and Ki67 ( 25%) and negative staining for CDX2, SOX10, Ck20, PAX8, p63, TTF1, CAIX, AE1/AE3, CK7, Melan A. Based on histologic features and immunostaining, this tumor is consistent with Renal Angiosarcoma.
Discussion:
Angiosarcomas are rare malignant tumors that arise from endothelial cells.
They account for <2% of soft tissue sarcomas and usually arise in skin and soft tissue and rarely in breast, liver, lungs, bone, spleen and very rarely in kidney.
Less than 70 cases of primary renal angiosarcomas have been reported in literature thus far.
Occurs in 6th and 7th decade, mean age is 61 years.
Etiology is unknown. Some of the risk factors mentioned in literature include chronic lymphedema, radiation, toxins such as arsenic and vinyl chloride, neurofibromatosis, BRCA1 and BRCA2 mutations, and familial syndromes such as Maffucci, Klippel–Trenaunay, and Stewart–Treves syndrome.
Clinically, patients present with flank pain, hematuria, abdominal mass and anemia. Sometimes, these masses are found incidentally.
The tumors are usually large, measuring from 3.7 to 30 cm in diameter, and are detected in advanced stages of the disease.
On imaging, these tumors show extensive hemorrhagic or necrotic mass with variable peripheral enhancement. CT findings can be similar to that of renal cell carcinoma.
Heo et al reported that T2-weighted imaging demonstrated a tangled mesh of tumor vessels with signal voids in the periphery of the mass, corresponding to the areas with strong enhancement on contrast-enhanced MRI.
Histologically these tumors are composed of anastomosing vascular spaces lined by malignant cells containing nuclei with pleomorphism and mitotic activity.
They are positive for endothelial cell markers like CD31, CD34, ERG, VEGF, FLI-1 and Factor VIII. The can also stain positive for vimentin and show high proliferation index on Ki67 staining. Tumor cells stain negative for Ae1/Ae3, CK7, CD10, HMB45, and EMA.
Renal cell carcinomas are positive for biomarkers such as CK7, alpha-methylacyl-CoA racemase (AMACR), carbonic anhydrase IX and transcription factor enhancer 3. Positive staining for CD31 and CD34 is also found in renal hemangioma. However, smooth muscle actin is detected in the myxoid supporting stroma cells of hemangioma but not in angiosarcomas.
Angiomyolipoma diagnosis is confirmed by the presence of positive reactivity for biomarkers such as melanocytic markers (Melan-A and HMB-45 antigen) and smooth muscle markers (smooth muscle actin and caldesmon) for which renal angiosarcomas stain negatively.
Epithelioid hemagioendotheliomas can look like angiosarcomas and can also stain positive for CD31, ERG and Ki67. But in angiosarcomas, the specificity of tumor cells is more obvious with more mitosis , larger tumor size and common hemorrhagic necrosis, common spindle cells in solid areas, and vascular cavities connected into a network region.
The best treatment option for primary renal angiosarcoma still remains unclear, and the most appropriate therapy is radical nephrectomy. Anti-angiogenesis factors like the vascular endothelial growth factor (VEGF) receptor blockers have been used successfully in the management of angiosarcoma recently.
The prognosis is very poor. In more than 70% of the reported cases, patient die within a mean interval of 7.3 months.
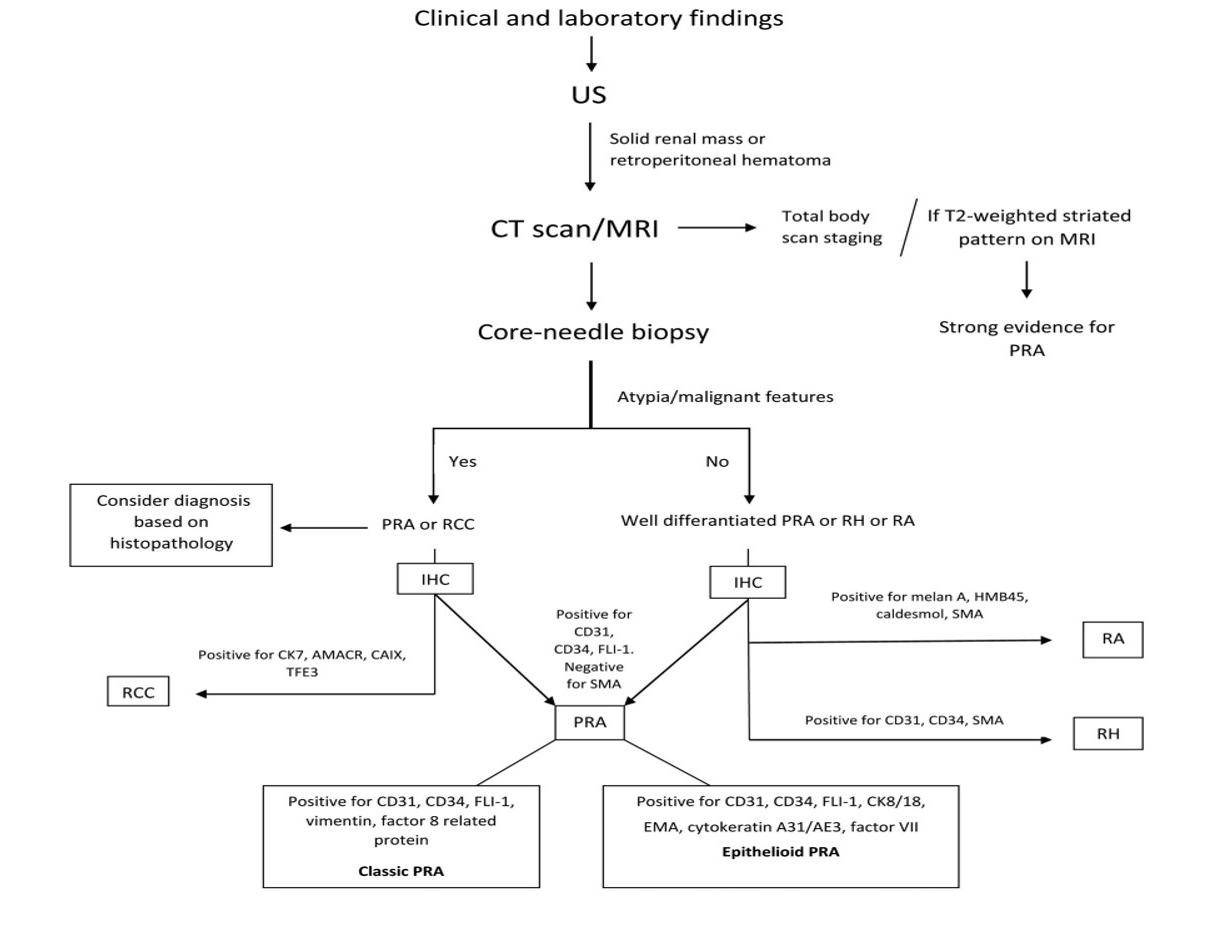
Figure 1. Diagnostic procedure for primary renal angiosarcoma. AMACR: Alpha-methylacyl-racemace; CAIX: carbonic anhydrase IX; CT: computed tomography; EMA: epithelial membrane antigen; FLI-1: friend leukemia integration 1; HMB45: human melanoma black 45; IHC: immunohistochemistry; PRA: primary renal angiosarcoma; RA: renal angiomyolipoma; RCC: renal cell carcinoma; RH: renal hemangioma; SMA: smooth muscle actin; TFE3: transcription factor enhancer 3
Mastoraki A, Schizas D, Giannakas T, Papadopoulos PP, Naar L, Vergadis C, Anastasiou I, Vassiliu P, Pikoulis E, Liakakos T. Primary Angiosarcoma of the Kidney: Literature Review of a Rare Nosologic Entity. Anticancer Res. 2020 Feb;40(2):625-633. doi: 10.21873/anticanres.13992. PMID: 32014903.
References:
Mastoraki A, Schizas D, Giannakas T, Papadopoulos PP, Naar L, Vergadis C, Anastasiou I, Vassiliu P, Pikoulis E, Liakakos T. Primary Angiosarcoma of the Kidney: Literature Review of a Rare Nosologic Entity. Anticancer Res. 2020 Feb;40(2):625-633. doi: 10.21873/anticanres.13992. PMID: 32014903
Omiyale, A.O., Carton, J. Clinical and Pathologic Features of Primary Angiosarcoma of the Kidney. Curr Urol Rep 19, 4 (2018). https://doi.org/10.1007/s11934-018-0755-6
Heo SH, Shin SS, Kang TW, Kim GE. Primary renal angiosarcoma with extensive hemorrhage. CT and MRI findings 2019;45:402-5.
Kazaz IO, Ersoz S, Colak F, Teoman AS, Kazaz SN, Karaguzel E, Kutlu O. Primary renal angiosarcoma: A case report and a short review of literature. Indian J Pathol Microbiol. 2020 Feb;63(Supplement):S44-S46. doi: 10.4103/IJPM.IJPM_66_19. PMID: 32108626.